OUR EQUIPMENT.
Currently in the ACT, our drug testing is undertaken using the Fourier-transform infrared spectroscopy (FTIR) using an ALPHA II machine. In addition to it’s technical appropriateness using FTIR provides pill testers with a level of legal protection as it removes the issue of contact with illicit substances by those conducting the test. As pill testing expands and authorities become more comfortable with the concept more detailed analysis will become possible.
Infrared spectroscopy (IR) is a presumptive test able to quickly identify ingredients by comparing the spectrum of the substances with known spectra in a library/ies.
IR is a highly discriminatory method and Harper, Powell and Pijl (2017:6) conclude that ‘when reference spectra it requires only a very small sample size in the range of milligrams or less. Additionally, samples can be studied in virtually any physical state…’. The World Health Organisation has stated that ‘infrared spectrum is not usually greatly affected by the presence of small quantities of impurities in the substance tested’ (WHO, 1997). Interference from moisture can occur but as the samples were being tested immediately this was regarded as a low risk. Importantly, samples required little preparation, so this eliminates ‘the possibility of sample contamination during sample preparation’ (Monit, 2007).
Test results are assigned to one of three outcomes. ‘Red’ results (drugs associated with increased harm/multiple overdoses/death) and some ‘yellow’ (disparity between result and patron expectation) and ‘white’ results (consistent with patron expectation) are displayed for all patrons, on similarly coloured pieces of paper, pinned to a notice board to alert patrons of what may be circulating.
Patrons are advised of the amnesty bin in which they could discard their drugs should they choose to do so.

Currently, with the technology we use, we can:
- Provide information on the ingredients of the drug presented.
- Provide advice on the predominant ingredient, and some minor ingredients
- Provide general advice on the potential effects and harms of any ingredients identified (done by medical staff and peer counsellors)
- Provide general advice on the reducing the chance of harm in the music festival environment, particularly in the context of illicit drug consumption
- Provide warnings regarding drugs (and ingredients) known to be associated with harm
- Provide warnings regarding drugs (and ingredients) that appear to be novel (unknown) to our very large database of drugs (and ingredients)
- Provide relevant information to festival healthcare personnel in real time
But we cannot:
- Provide exact information on the purity or doses of ingredients within the drugs presented
- Guarantee that we can identify all products, particularly ‘unknown unknowns’
We will not:
- Tell anyone that their drugs are ‘safe’
- Tell anyone that their drugs are ‘good’
- Provide a moral judgement on the behaviour of service users
UPLC-PDA System (Fixed site only)
Pill Testing Australia (PTA) working with The Australian National University (ANU) and analytical partners Waters Australia have developed rapid Ultra-Performance Liquid Chromatography-Photodiode Array (UPLC-PDA) analysis for pill testing applications. This machine has been central to the work the CanTEST Health and Drug Checking Service conducted in the ACT and has been used to analyse over 400 samples during the six month pilot period. The method has also been published by Waters as an application note on its website.
The UPLC-PDA analysis can test the purity and identity of ten targeted drugs (Figure 1) in under three minutes. This rapid analysis can be used to test substances and provide information directly to clients attending pill testing services. The machine can also detect the presence of adulterants in drug samples thereby alerting clients to the presence of unknown and potentially harmful contaminants. Over the course of the pilot, the team of qualified analysts has expanded the number of substances that can be identified by the instrument to include a range of new psychoactive substances appearing unexpectedly in the unregulated drug market. Methods now include routine analysis for the presence of the ketamine derivative 2’-fluoro-2-oxo-PCE (also known as CanKet) that is frequently presented as ketamine in the ACT. To our knowledge CanTEST was the first drug checking service anywhere in the world to identify and routinely analyse for this substance. Methods also include the capacity to detect the presence of the new synthetic opioid drug metonitazene, a member of the nitazene family of drugs which have been the subject of public health alerts in ACT and NSW in late 2022.
The UPLC-PDA analysis uses liquid chromatography to separate drug components which are then detected by ultra-violet visible spectroscopy. The analysis results are compared to a library of data to verify the drug identity. The signal intensity can be calibrated to establish drug purity with the method being accurate and precise across a range of expected drug concentrations.
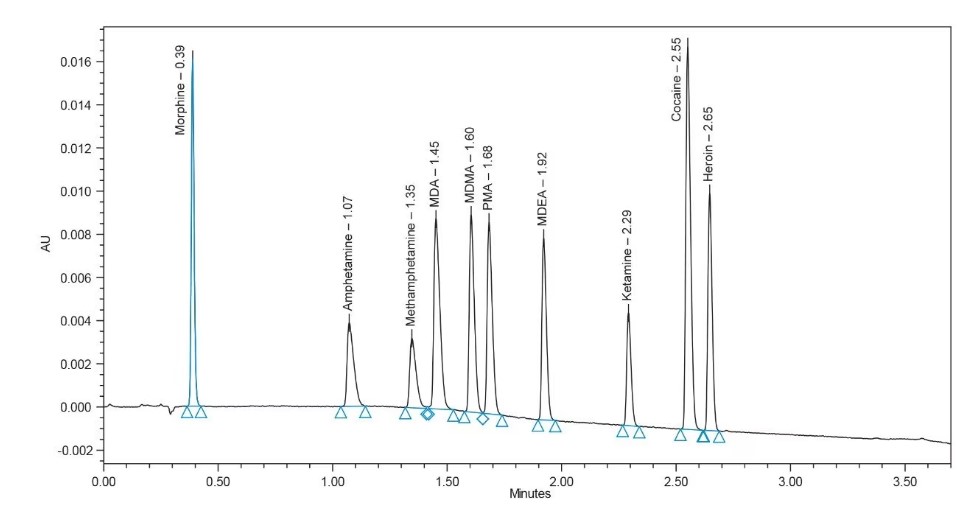
Figure 1: UPLC-PDA chromatogram showing the separation and detection of ten targeted drugs in under three minutes.
Relationship to Alternative Analytical Methods
The method complements the Fourier Transformed Infra-Red (FTIR) analysis used by PTA in Australia’s only two government-sanctioned, front-of-house pill testing trials held earlier in the ACT and also operated at the CanTEST service. The FTIR analysis can presently identify major drug components for a broader range of substances but cannot provide information on drug purity. The UPLC-PDA analysis can detect targeted drugs at lower levels and give purity information.
Longer term, the UPLC-PDA instrument can be upgraded to incorporate mass spectrometry (MS) detection. This mode of detection is already supported by the instrument software, as detailed in the application note. The addition of MS detection would further improve the ability to identify sample contaminants occurring at lower proportions of the drug samples.

